The UMaine Student Symposium is an annual event featuring research presentations from undergraduate and graduate students, and is a way to share student research on campus and with the Maine public.
All of the abstracts for the full program, and previous years, are available here.
The event is free to attend, and will take place at the New Balance Field House on the UMaine Orono Campus, Friday April 12, 2024.
- 8:00 a.m.: Doors open
- 8:15 a.m.: UMaine Flute Ensemble
- 9:00 a.m.: Opening Remarks
- 9:30-11:30 a.m.: Graduate Poster / Oral / Exhibit Presentations
- 11:00 a.m. – 1:00 p.m.: Undergraduate Poster / Oral / Exhibit Presentations
- 9:15 a.m. – 10:30 a.m. – Musical Performances at Minsky Recital Hall, Class of 1944 Hall
- 1:00 -2:00 p.m.: Student Panel
- 2:00 p.m.: Keynote Speaker, Sreeram “Ram” Dhurjaty, PhD
- 2:45 p.m.: Free Parking, Jazz Performance
- 3:15 p.m.: Awards Ceremony and Closing Remarks
Several students from the Ishaq Lab will be presenting their ongoing work:
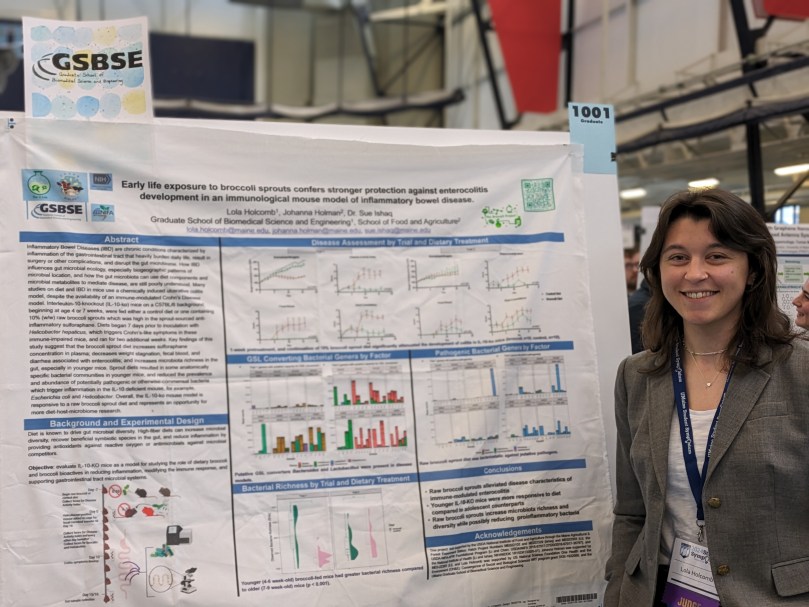
Early Life Broccoli Sprout Consumption Confers Stronger Protection Against Enterocolitis in an Immunological Mouse Model of Inflammatory Bowel Disease

Author(s): Lola Holcomb, Johanna Holman, Sue Ishaq.
Type: poster presentation
Submission category: Biomedical sciences
Abstract number 1001: Inflammatory Bowel Diseases (IBD) are chronic conditions characterized by inflammation of the gastrointestinal tract that heavily burden daily life, result in surgery or other complications, and disrupt the gut microbiome. How IBD influences gut microbial ecology, especially biogeographic patterns of microbial location, and how the gut microbiota can use diet components and microbial metabolites to mediate disease, are still poorly understood. This study aimed to resolve such questions. Many studies on diet and IBD in mice use a chemically induced ulcerative colitis model, despite the availability of an immune-modulated Crohn’s Disease
model. Interleukin-10-knockout (IL-10-KO) mice on a C57BL/6 background, beginning at age 4
or 7 weeks, were fed either a control diet or one containing 10% (w/w) raw broccoli sprouts
which was high in the sprout-sourced anti-inflammatory sulforaphane. Diets began 7 days prior to inoculation with Helicobacter hepaticus, which triggers Crohn’s-like symptoms in these immune-impaired mice, and ran for two additional weeks. Key findings of this study suggest that the broccoli sprout diet increases sulforaphane concentration in plasma; decreases weight stagnation, fecal blood, and diarrhea associated with enterocolitis; and increases microbiota richness in the gut, especially in younger mice. Sprout diets resulted in some anatomically specific bacterial communities in younger mice, and reduced the prevalence and abundance of potentially pathogenic or otherwise-commensal bacteria which trigger inflammation in the IL-10 deficient mouse, for example, Escherichia coli and Helicobacter. Overall, the IL-10-KO mouse model is responsive to a raw broccoli sprout diet and represents an opportunity for more diet-host-microbiome research.
Steamed Broccoli Sprouts Alleviate Gut Inflammation and Retain Gut Microbiota Against DSS-induced Dysbiosis.

Author(s): Johanna Holman, Lola Holcomb, Sue Ishaq.
Type: oral presentation, 9:45 am
Submission Category: Biomedical Sciences
Abstract number 1002: Inflammatory bowel diseases are devastating conditions of the gastrointestinal tract with limited treatments, and dietary intervention may be effective, affordable, and safe for managing symptoms. Research has identified inactive compounds in broccoli sprouts that may be metabolized by the gut microbiota into key anti-inflammatories. Our research set out to identify biogeographic locations of participating microbiota and correlate that to health outcomes. We fed specific pathogen free C57BL/6 mice either a control diet or a 10% steamed broccoli sprout diet, and gave a three-cycle regimen of 2.5% dextran sulfate sodium in drinking water over 40 days to simulate ulcerative colitis. We monitored body weight, fecal characteristics and lipocalin, and sequenced bacterial communities from the contents and mucosa of the jejunum, cecum, and colon. Mice fed the broccoli sprout diet while receiving dextran sulfate sodium performed better than mice fed control diet for all disease parameters, including increased weight gain (2-way ANOVA, p < 0.05), lower Disease Activity Index scores (2-way ANOVA, p < 0.001), and higher bacterial richness (linear regression model, p < 0.01). Bacterial communities were assorted by gut location except in the mice receiving the control diet and colitis-inducing treatment (Beta-diversity, ANOVA, p < 0.05). Importantly, our results suggest that broccoli sprouts abrogated the effects of dextran sulfate sodium on the gut microbiota, that colitis erases biogeographical patterns of bacterial communities, and that the cecum is not likely to be a contributor to colonic bacteria of interest, in a mouse model of ulcerative colitis.

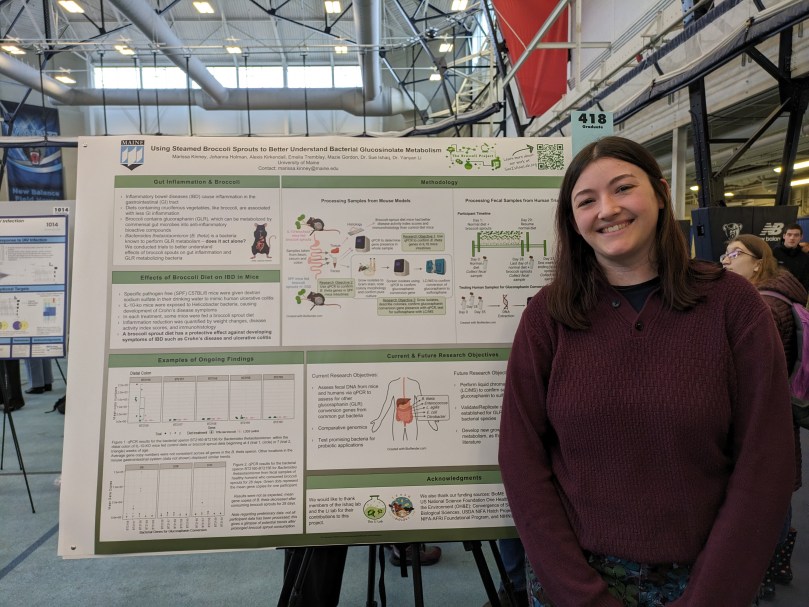
Using Steamed Broccoli Sprouts to Better Understand Bacterial Glucosinolate Metabolism

Author(s): Marissa Kinney, Johanna Holman, Alexis Kirkendall, Emelia Tremblay, Mazie Gordon.
Type: poster presentation
Submission Category: Allied Health
Abstract number 418: Inflammatory bowel diseases (IBD) lead to dysfunction of the gastrointestinal (GI) tract, resulting in disruption to overall health. These diseases can affect people of all ages and are present on a global scale. Research has demonstrated that diets high in cruciferous vegetables, such as broccoli, are associated with decreases in GI inflammation. Broccoli contains glucoraphanin, which through metabolism by gut bacteria, can become an anti-inflammatory compound, sulforaphane. Recent research has validated the use of steamed broccoli sprouts in the diet of mice to reduce inflammation and resolve symptoms of IBD. Isolated microbiota samples obtained from various locations in the GI of these mice are being investigated for the presence of glucoraphanin-metabolizing genes from a common gut bacteria, Bacteroides thetaiotaomicron (B. theta). Similar analyses being conducted on human fecal samples from individuals who consumed steamed broccoli sprouts for 28 days have demonstrated decreases in the presence of B. theta. This result was not anticipated and has strengthened beliefs that B. theta is not the primary species performing glucoraphanin metabolism, thus prompting further analyses of the fecal samples from mice and humans for glucoraphanin-metabolizing genes of other common GI bacteria. Genomes of isolates from the gut of mice which have high quantities of glucoraphanin-metabolizing genes will be sequenced for identification. This information will help to identify potential bacterial candidates for future research on probiotic development.